
Commercializing the Zinc or Graphite Battery in Grid-Scale Energy Storage
Grid-scale renewable energy has been steadily increasing, and, therefore, several researchers have moved their attention from producing energy to the storage of energy.
The sources for producing renewable energy are intrinsically variable, such as solar cells that transform sunlight into power, or windmills that convert air currents into electrical currents.
Still or extremely cloudy days can cause obvious fluctuations in the power output of renewable sources. A significant obstacle for grid-scale renewable energy is to find ways for efficient storage of such usually intermittent energy sources for later and more evenly distributed use.
Under the guidance of Professor CUI Guanglei and ZHAO Jingwen from Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), the Chinese Academy of Sciences (CAS), a team of researchers is one step closer to finding a solution to this storage issue.
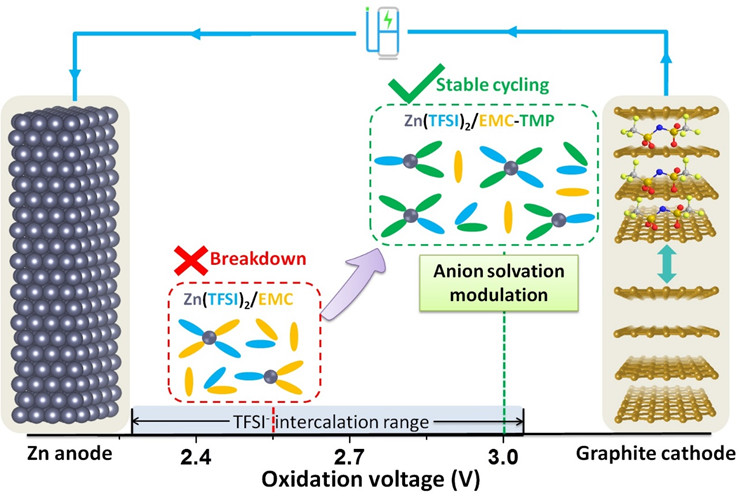
The focus of the team has been on graphite cathode and zinc anode-based dual-ion batteries (DIBs) as they are inexpensive and exhibit high voltage abilities, which makes them a potential candidate for huge, grid-scale storage devices. The study results were published in Angewandte Chemie’s international edition on August 18th, 2020.
In DIBs, Zn deposition takes place on a Zn anode as part of the charging process, whereas the negatively charged anions bind themselves into the graphite cathode, which enables energy storage for later use. This process is called intercalation. But zinc/graphite batteries have a drawback.
Most importantly, the electrolytes utilized to shift charge via the battery would experience oxidative decomposition at high voltage regions, which decreases the efficiency and the service life of the battery. This is a crucial issue to resolve before this kind of battery is used for large-scale power storage.
The research team’s new method improved the oxidation stability of carbonate-based electrolytes by modifying the anion solvation structure. The researchers added a strongly-electron donating trimethyl phosphate (TMP) solvent and could capture the anions in the TMP solvation regime and dissociate the anions from carbonate solvent.
As a result, the operating voltage of the zinc/graphite batteries was increased by 0.45 V while also increasing the service life (92% capacity retention following 1000 cycles). This could not just increase the service life of zinc/graphite batteries but also improve the capacity of anion intercalation into graphite. The authors stressed that “a deep understanding and regulation of the anion solvation structure is essential.”
The focus of their next study will be on improving the energy density, inhibiting the self-discharge behavior, and reducing the cost of the electrolyte for Zinc/Graphite batteries. The ultimate aim is to achieve commercialization of the zinc/graphite battery in grid-scale energy storage.













